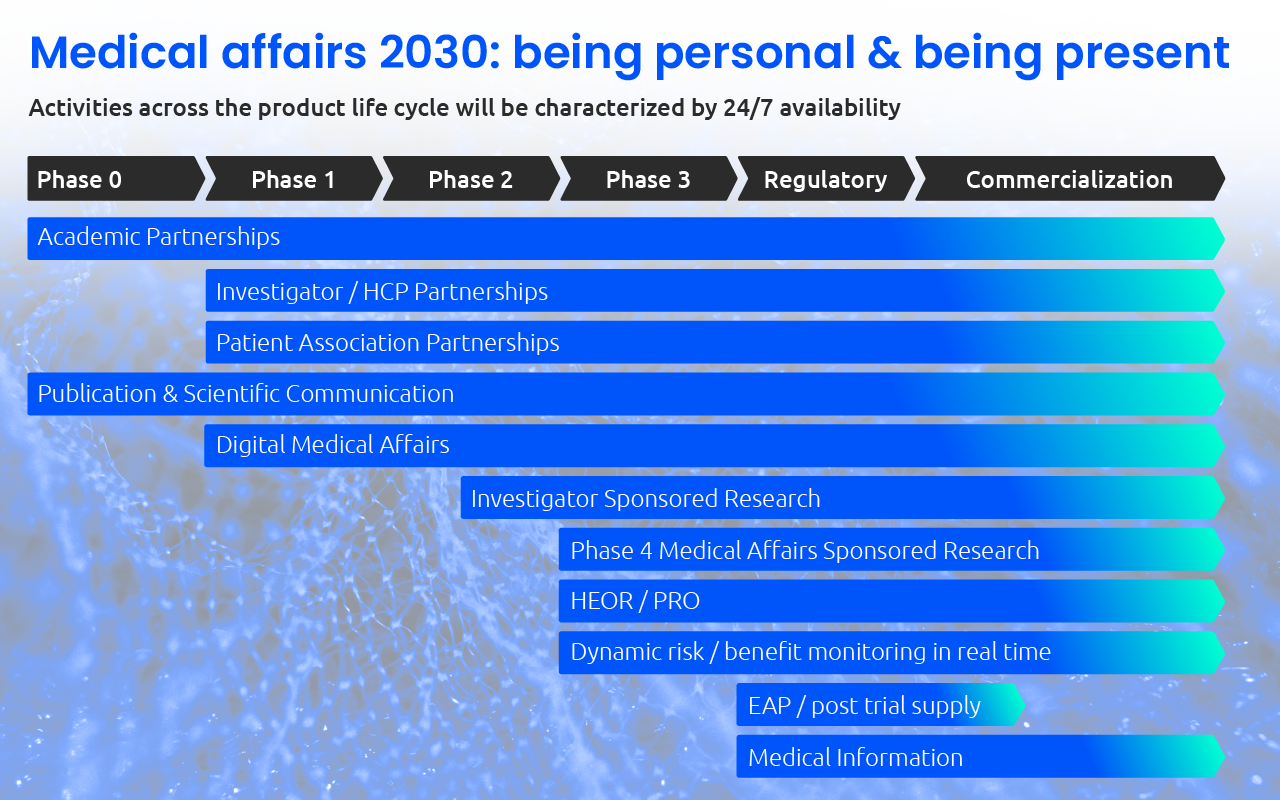
Medical Affairs 2030:
holistic real-world data collection and knowledge generation
As previously discussed, the medical affairs “currency of the future” will be partnership and trust and will extend into new ways of data collection and knowledge generation. They will go beyond the traditional interventional or observational studies and use holistic real-world evidence, by collecting any available and accessible data in real time. This will allow for pattern recognition through AI and create a new state of the art. Possibly, this can also address the challenge that patients may not be able to communicate signs and symptoms or describe their treatment experience at the level of detail needed.
Whilst today’s data is collected with the perspective of evaluation at a future point in time, the ability to monitor treatment in real time and react as soon as evidence is available, is becoming a reality. This is only possible in partnership with HCPs and patients. Such information will help guide patients to receive the right treatment at the right time with the best possible benefit/risk balance; in other words safe & appropriate use.
The way in which new treatment options are being introduced needs to change. Today regulators expect the highest possible level of confidence in safety and efficacy of a new treatment option as a condition for approval. The gap between conducting a clinical trial exposing a patient to a new treatment under controlled conditions and allowing HCPs to prescribe an approved medication can be long. Often this is at the expense of patients as it delays access to promising treatment options. Time that many patients don’t have.
Picture description
Is it possible to imagine that a new treatment option is introduced in a controlled environment similar to a clinical trial using real time monitoring and real-world evidence strategies?
The answer is yes. Certainly, a complex task, but one that companies, and medical affairs in particular need to live up to.
The way patients should be monitored post launch needs to be piloted during the pivotal trial, so that it becomes a seamless transition with comparable data sets allowing for real time monitoring of benefit/risk ratios. If HCP and patients want access to a new treatment option that promises a significant benefit over established standard of care, they will have to agree to this monitoring which should be as automated and effortless as possible.
Proposing such strategies the question will come up, if an “unstructured” data collection puts a successful development and approval at risk. From an ethical and patient perspective … the earlier challenges and potential risks are identified the better so they can be managed and overcome.
If you are interested to discuss and/or workshop what your medical affairs department could look like in the future, please contact me via info@infill.com
Author