Not just numbers: the rise of patient-reported outcomes in clinical research
The fundamental goal of clinical trials in oncology is to develop drugs that are safe and that effectively address patients’ key symptoms, prolong life and increase overall wellbeing. Clinical trials are mostly based on objective metrics such as tumor size or survival rates, that help generate robust data on a drug’s efficacy. Unfortunately, not all data are as easy to collect as the 5-year survival rate, but they are nevertheless of crucial importance. When dealing with patients other issues often play a role as well, as every physician knows to report. How patients experience a medical intervention and how it affects their life beyond biomarkers must be taken into account if medical research is to put patients’ interests in the center of its mission.
To bridge the gap between hard data and empathetic patient care, the importance of patient-reported outcomes (PROs) and quality of life (QoL) data in clinical trials has been increasingly recognized. “How are we doing today?” is probably one of the most frequently asked questions in hospitals, but this personal approach was not systematically incorporated into clinical studies in the past. Generally speaking, PROs are any report of the status of a patient’s health condition that comes directly from the patient. They can include symptoms, function, health-related quality of life, and satisfaction with treatment. QoL encompasses a patient’s physical, psychological, and social well-being.
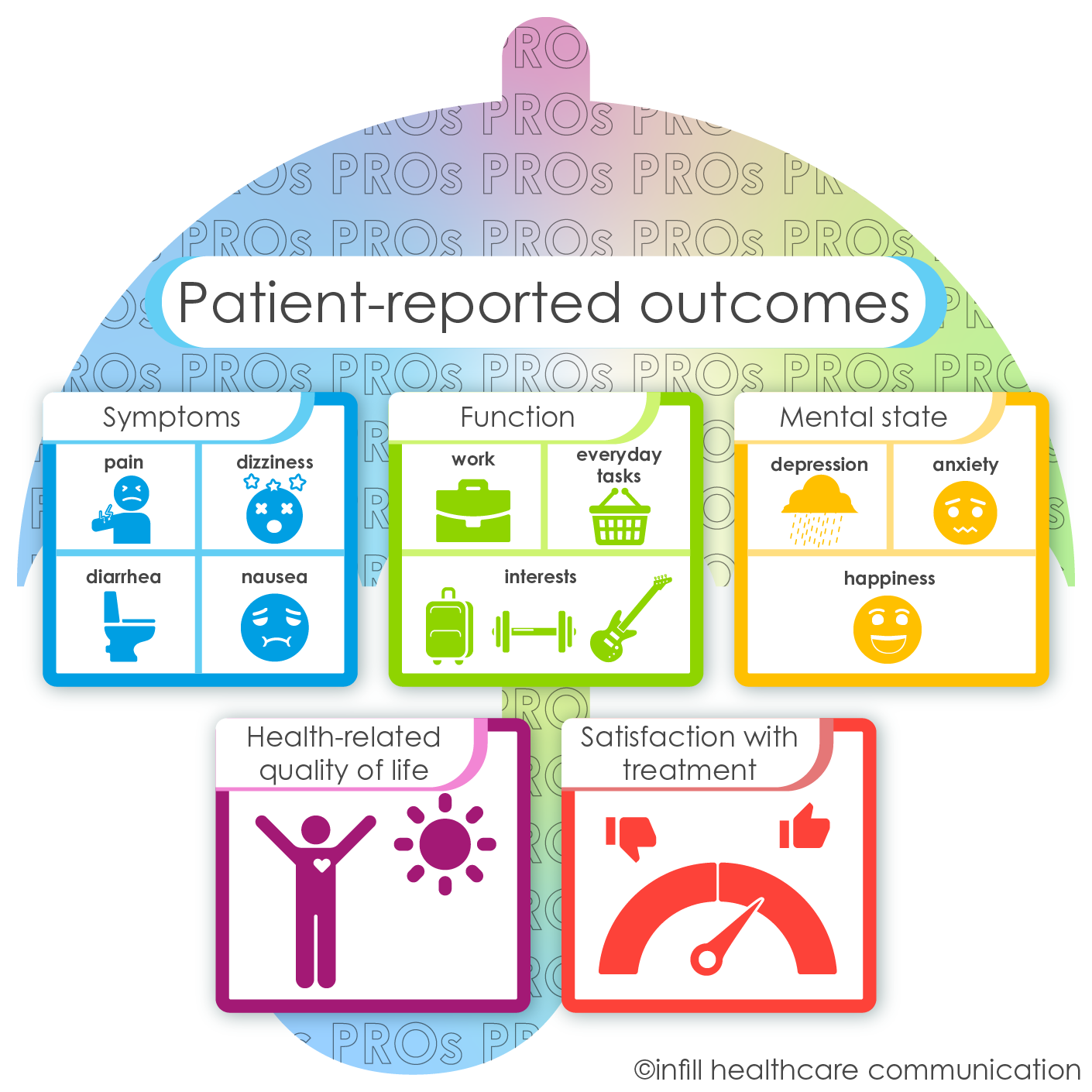
An overview of possible categories for patient-reported outcomes
In oncology, for example, PROs are being used to evaluate the impact of cancer treatments on patient symptoms, function, and overall well-being. Pre-treated patients and patients in later stages of disease want the side effects to be in reasonable proportion to the efficacy of a drug. PROs and QoL data can be used to identify potential side effects and other issues that may arise during treatment. For study participants, this also means that more time and effort is devoted to hearing their perspective.
The FDA and EMA now strongly encourage sponsors of clinical trials to include PROs and QoL data in their submissions. This helps ensure that new treatments are evaluated not only on their clinical efficacy but also on their impact on patients’ lives.
In conclusion, PROs and QoL data are increasingly recognized as essential components of clinical research. They provide valuable insights into the patient experience and can help inform treatment decisions, regulatory decision-making, and healthcare policy. As such, they will continue to play an important role in improving patient outcomes and overall healthcare quality.